Even in healthy individuals, the list of side effects that have been reported for the various fat burners is long (Salacinski et al. highlight G.I. distress, and liver inflammation, which may accompany weight loss resulting from chronic supplementa-tion, in particular)... and while it is long, very long, in fact, it is hardly science based, because evidence controlled treatment trials in healthy and diseased patients are rare, insufficient or simply lacking. That's bad news. After all, for most currently marketed OTC weight loss agents' kitchen-sink approach to fat loss, there's no evidence that they even work.
If you're looking for a true fat burner, try coffee ;-)
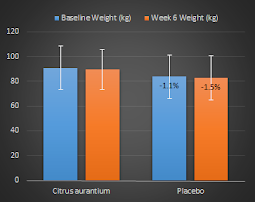
As a more recent study by Urbina et al. (2012) appears to suggest, it doesn't necessarily take ephedrine, though, to elicit significant increases in metabolic rate. The ephedrine-free caffeine + green tea based successor to the ephedrine-based fat burner DymaBurn(TM) may not be as effective, but can still elevate the resting metabolic rate of 6 male and 6 female subjects (N = 12, 22 ± 9.5 yrs, 171 ± 11.2 cm, 76.9 ± 11.2 kg, 22.7 ± 9.5), who consumed either a 2 capsule serving of Dyma-Burn Xtreme (DBX) or placebo (PLC), significantly (see Figure 2):
Fat burners are no magic weight loss pills and still, they can help you lose weight - especially the stim-based ones - because they may reduce appetite, increase energy and thus your ability to adhere to an energy restricted diet with or without concomitant exercise.
The placebo (control trial) was always ingested on the morning of the first measurement session two hours prior to measurements. The supplement (R or MAB) taken upon awakening prior to the second and third measurement session was randomly determined by a coin toss, again two hours before the data collection. Whichever supplement was not taken prior to the second measurement trial was taken prior to the third measurement trial. At least 24 hours separated the first session (control) with the second session; and at least 48 hours separated the second session from the third session to minimize interference from the previous supplement."As recommended by Compher and colleagues (2006), subjects were instructed to refrain from exercising for 24 hours and from consuming food for 12 hours prior to testing. The participant ingested a placebo (an empty digestible capsule) or a dose of one of two supplements; CelluCor CLK or raspberry ketones (R) and CelluCor T7 or the metabolic activator blend (MAB) with water, upon awakening on the morning of each measurement session. The three softgel and two softgel tablets were considered to be a single dose of R and MAB, respectively, as recommended by the manufacturer.
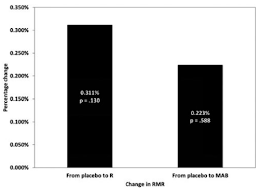
Figure 3: Changes in RMR after the administration of raspberry ketones or CelluCor CLK; p > 0.05 for both (Salacinski. 2016).
R was composed of 1.7 g of conjugated linoleic acid, 500 mg of l-carnitine tartrate, 100 mg of R, and 100 mg of 7-ketodehydroepiandrosterone. The MAB product consisted of 494 mg of MAB with an ingredient list of white willow bark, cayenne, 3-iodotyrosine, 3, 5-diiodotyrosine, 200 mg of zinc arginate chelate, 150 mg of sea weed extract. 66 mg of niacinamide, 66 mg of griffonia seed extract, and 0.2 mg of selenium" (Salacinski. 2016).
The results of this recent rel. small scale (N=26, no dropouts) study are plotted in Figure 3. Data from two participants were excluded from the statistical analysis because the data could not be adjusted to meet the acceptable criteria recommended by Compher et al. (2007) and Frankenfield et al. (2003). Despite the two exclusions, it should be obvious that the non-significant 0.111% increase due to raspberry ketones and the even lower increase due to CelluCor CLK are meaningless.
Irrespective of any methodological gaps, the study at hand suggests shows one thing with a decent certainty: if raspberry ketones alone or RK blends like CelluCor CLK, which contains hydrolysates of Blue Whiting, L-Carnitine Tartrate, CLA (Conjugated Linoleic Acid) and Razberi-K®, a proprietary raspberry extract marketed as "the original ketone ingredient behind this movement", work at all - it is not by increasing your REE | Comment!
- Anderson, Richard A. "Chromium, glucose intolerance and diabetes." Journal of the American College of Nutrition 17.6 (1998): 548-555.
- Bent, Stephen, Amy Padula, and John Neuhaus. "Safety and efficacy of citrus aurantium for weight loss." The American journal of cardiology 94.10 (2004): 1359-1361.
- Colker, Carlon M., et al. "Effects of Citrus aurantium extract, caffeine, and St. John's wort on body fat loss, lipid levels, and mood states in overweight healthy adults." Current Therapeutic Research 60.3 (1999): 145-153.
- Greenway, Frank L., et al. "Effect of a Dietary Herbal Supplement Containing Caffeine and Ephedra on Weight, Metabolic Rate, and Body Composition*." Obesity research 12.7 (2004): 1152-1157.
- Molnar, D., et al. "Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents." International Journal of Obesity 24.12 (2000): 1573-1578.
- Onakpoya, Igho J., et al. "The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials." European journal of nutrition 51.2 (2012): 127-134.
- Salacinski, Amanda J., et al. "The Acute Effects of Nonstimulant Over-the-Counter Dietary Herbal Supplements on Resting Metabolic Rate." Journal of dietary supplements (2015): 1-10.
- Stickel, Felix, Eleonora Patsenker, and Detlef Schuppan. "Herbal hepatotoxicity." Journal of hepatology 43.5 (2005): 901-910.
- Urbina, Stacie, et al. "Effects of ingesting Dyma-Burn Xtreme, a thermogenic dietary supplement on metabolic rate and subjective measures of mood state." JISSN 9.Suppl 1 (2012): P31.