With that being said, let's take a look at what the scientists did to "investigate the role of MC4R in the modulation of muscle work efficiency, and test the hypothesis that energy restriction alters economy of activity through decreasing the response to central activation of MC4R" (Almudarij 2017).
You can find more diet related wisdom in the True or False articles at the SuppVersity
These rats were subjected to 3 weeks of 50% calorie restriction (CR). Over the course of this - in rodent years - intermediate time period, the scientists assessed their lab animals resting and nonresting energy expenditure (EE) and calculated the total, as well as the activity-associated EE, muscle thermogenesis, and sympathetic outflow.
Dieting is when you leave only 360kcal not 600kcal in the gym, despite doing the same workout
What is particularly interesting, yet often forgotten when we talk about dieting (especially within the fitness community), is the fact that the energy that you will burn during exercise will also decrease significantly (see Figure 1G). One of the implications of the study at hand we cannot ignore is that the reduced physical activity energy expenditure stems from "the dampening of both the amount and energetic cost of activity" (Almundarij 2017) - and the latter, i.e. the reduced energy expenditure in response to a standardized exercise regimen amounts to a 30-40% decrease in EE that would degrade the 600kcal you believe to be burning on the treadmill to a meager 360-420 kcal/session!
 |
| Figure 2: Fat & lean mass and the rel. (%) difference in body comp. w/ ad-libitum vs. restricted diet (Almundarij 2017). |
 |
| Illustration of the allegedly over-simplified example calculation to show the significance of the fasting-induced reduction in AIEE for meal timing and fat loss as observed in Garaulet 2013. |
Let's illustrate that with a simple example (see Figure to the left). Let's assume the reduction in AIEE is indeed 40%. Let's further assume that you'd "burn" ~1000kcal from working out and walking in your waking phase before the PM meal and only 150kcal after the PM meal when eating an energetically balanced. According to Cooker, that would put your effective AIEE while dieting to 600kcal + 150kcal when you eat in the PM, but 1000kcal + 90kcal if you eat the meal in the AM. Obviously, this oversimplified example assumes that the metabolism would not slow down over the day (which will be the case). Eventually, the difference will thus certainly be smaller (maybe 15% instead of the 31% in my example). That does not mean, though, that it could not still be statistically and practically significant (note: it is unlikely that a relevant reduction would be observed for intermittent fasting in the absence of a significant caloric deficit).
is the first report of reduced muscle NETO [norepinephrine turnover], indicating lower SNS drive to skeletal muscle after 3 weeks of food restriction (Fig. 2), an effect not seen during short-term energy restriction (Dulloo et al. 1988)" (Almundarij 2017).With the importance of skeletal muscle to both resting and activity EE, (Zurlo et al. 1990; Gallagher et al. 1998), "this low SNS drive" could, as the authors further point out significantly "contribute to both the resting and nonresting aspects of adaptive thermogenesis" (Almundarij 2017).
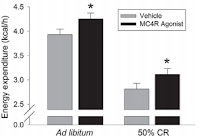 |
| Figure 3: The MC4R induced increase in energy expenditure in the study at hand is probably not coincidentally of a similar magnitude as the effects of nicotine (Almundarij 2017). |
"[...] provide potential avenues to counter adaptive thermogenesis and [thus to] promote continued weight loss and weight maintenance through targeting physical activity EE and skeletal muscle thermogenesis (Almundarij 2017).Now the bad news is that the melanocortin 4 receptor agonists Almundarij et al. used in their study are not (yet?) ready to be used in human beings. Other tools to increase the decreased norepinephrine turnover in skeletal muscle, however, are available and you'll all be familiar with their names: caffeine or ephedrine (and to a lesser extent green tea extract).
 |
| Figure 5: There is a link for nicotine and there may even be a link of caffeine to the melanocortin 4 receptor - one that's mediated by the POMC neurons. |
In a different context this relationship has already been established (Bhorkar 2014), whether and to which extent caffeine stimulates the melanocortin 4 receptors (MC4R), however, is - at least as far as I know - not known. Anyway... when all is said and done, there's still no doubt that caffeine, even when it's used alone, will still have a significant enough effect on the sympathetic nervous system (SNS) to promote weight loss and weight maintenance in multiple diet studies (Dulloo 1989; Westerterp‐Plantenga 2005) - and let's be honest: many people won't even care if that involves an increase in MC4R activity or not ;-)
 |
| If your diet of choice is a ketogenic diet, caffeine will not just help you to compensate the reduction in exercise-induced energy expenditure and thus "restore the calories" you leave in the gym. A recent study shows that it will also help you to get and stay in ketosis - and that's even when you've been cheating on carbs | learn more. |
Now, this effect reflects in a reduced central activation of hypothalamic melanocortin receptors, which could be countered by medical intervention only theoretically. After all, corresponding drugs as they have been used for experimental purposes on the rodents in the study at hand are still in the early experimental phase - that they do work without short-term side-effects has yet been demonstrated in obese individuals by Chen et al. (2015) who observed a 111 kcal/24 h increase in REE.
For the average gymrat, these drugs will yet probably never be available legally. Against that background you can count yourselves lucky that Almundarij et al.'s results also point to another, already available and (if used sensibly) perfectly safe class of drugs. central nervous stimulants like the ubiquitous caffeine. These agents have a proven record of being able to promote diet-induced fat loss by increasing/restoring SNS-induced thermogenesis (Dulloo 1988 & 1989) - especially when used in conjunction with exercise so that they can partly compensate the diet-induced reduction in sympathetic tone and thus restore the significantly reduced energy expenditure during workouts to near-normal levels.
 |
| It is often belittled, but even in non-dieting humans, the increase in energy expenditure following the consumption of caffeine is significant (Astrup 1990). |
- Almundarij, Tariq I., Chaitanya K. Gavini, and Colleen M. Novak. "Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction." Physiological Reports 5.4 (2017): e13171.
- Astrup, A., et al. "Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers." The American journal of clinical nutrition 51.5 (1990): 759-767.
- Berkowitz, Barry A., James H. Tarver, and Sydney Spector. "Release of norepinephrine in the central nervous system by theophylline and caffeine." European journal of pharmacology 10.1 (1970): 64-71.
- Bhorkar, Amita A., et al. "Involvement of the central melanocortin system in the effects of caffeine on anxiety-like behavior in mice." Life sciences 95.2 (2014): 72-80.
- Bracco, David, et al. "Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women." American Journal of Physiology-Endocrinology and Metabolism 269.4 (1995): E671-E678.
- Chen, Kong Y., et al. "RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals." The Journal of Clinical Endocrinology & Metabolism 100.4 (2015): 1639-1645.
- Dulloo, A. G. "Stimulation of thermogenesis in the treatment of obesity: A rational approach." Journal of obesity and weight regulation (USA) (1988).
- Dulloo, A. G., et al. "Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers." The American journal of clinical nutrition 49.1 (1989): 44-50.
- Gavini, Chaitanya K., et al. "Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis." American Journal of Physiology-Endocrinology and Metabolism 306.6 (2014): E635-E647.
- Garaulet, Marta, et al. "Timing of food intake predicts weight loss effectiveness." International journal of obesity 37.4 (2013): 604-611.
- Laurent, Didier, et al. "Effects of caffeine on muscle glycogen utilization and the neuroendocrine axis during exercise 1." The Journal of Clinical Endocrinology & Metabolism 85.6 (2000): 2170-2175.
- Leibel, Rudolph L., Michael Rosenbaum, and Jules Hirsch. "Changes in energy expenditure resulting from altered body weight." New England Journal of Medicine 332.10 (1995): 621-628.
- Magkos, Faidon, and Stavros A. Kavouras. "Caffeine and ephedrine." Sports Medicine 34.13 (2004): 871-889.
- Mineur, Y. S., Abizaid, A., Rao, Y., Salas, R., DiLeone, R. J., Gündisch, D., ... & Picciotto, M. R. (2011). Nicotine decreases food intake through activation of POMC neurons. Science, 332(6035), 1330-1332.
- Mountjoy, Kathleen G. "Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes." Biochemical Journal 428.3 (2010): 305-324.
- Westerterp‐Plantenga, Margriet S., Manuela PGM Lejeune, and Eva MR Kovacs. "Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation." Obesity 13.7 (2005): 1195-1204.







