 |
| What's the verdict on sucralose? |
Today, sucralose, which has been pulled from the diet drinks of the biggest players in the beverage industry, i.e. Pepsi and Coke (allegedly because people didn't like it - conspiracy theorists listen up ;-), is still used in selected diet drinks, diet sodas, flavored iced teas, fruit-flavored waters, hot chocolate, as well as various sweetened condiments, dairy products, chewing gums, candy and your supplements (e.g. 30-60mg in 50g whey or casein).
You can learn more about sweeteners at the SuppVersity
 |
| Table 1: Click here to open the very long list of common products and the sweeteners they contain. Among the items that contain sucralose, you will find foods/beverages you wouldn't expect there, like oats or ketchup and several dozens of "sugar-free" products (Spencer 2016). |
The total daily intake in Romo-Romo's study equaled 15% of the Acceptable Daily Intake (ADI | learn more about the safety of sucralose in Grotz and Munro 2009). For sucralose that's (15 mg/kg/d) or 168mg/d for someone weighing in at 75kg.
Since the authors decided that the subjects should get their daily dose of sucralose from Splenda®, this means that the average subject, whose weight was 58kg (there were more female than male subjects in both groups 24/33 & 25/33, hence the low mean body weight of the average study participant) had to use a total of 11 packets of Splenda® (12mg sucralose, each) to arrive at their target dose of 130.5mg/d.
But let's leave the crazy fitness- and BB-crowds aside and take a look at "normal" people. Unfortunately, there are limited data on the consumption sucralose worldwide. The International Food Information Councilestimates the average daily sucralose intake of American sweetener connoisseurs at ~1.3mg/kg (8.67% of the ADI), which is 42% less than the 2.25mg/kg dose Romo-Romo et al. (2018) had the initially 33 subjects in the sucralose group consume.
Studies from other countries support the notion that the dosage the scientists used is not representative of the average sucralose consumption: Buffini et al. (2018) quantify the sucralose intake in Ireland at 2.51% (4.45% if only sweetener consumers are considered), and Ha et al. (2013) estimate the sucralose intake of the average Korean citizen at 8% of the ADI, i.e. ~1/3 and ~1/2 of the dosage used in the study under review. A very recent review by Martyn et al. (2018) who reviewed the published data on the intake of all major low-/no-calorie sweeteners in their article in the March issue of Nutrients adds data for another 7 countries (see table below) - all showing that the average consumption of adults is below the 15% of the ADI line.
Martyn 2018). For many other countries, only total sweetener intakes and/or the individual intake of other sweeteners were available.
Against that background, you will have a hard time arguing that the study results would apply 1:1 on a population level, worldwide. On an individual level, this looks different, though. For high consumers, as well as toddlers and younger children (sucralose has also been found in breast milk), the results of the study at hand are thus very relevant.
Are there any methodological problems that raise serious doubts about the accuracy or practical relevance of the results of the study under review?
The subjects in both arms of the study, i.e. the active treatment arm in which the subjects consumed 2.25mg/kg sucralose per day in form of Splenda® sachets, the contents of which they emptied into the beverages they consumed with breakfast, lunch, and dinner, and the control arm, followed the same procedures and had to adhere to the same dietary/behavioral principles, namely:
"Both groups were instructed to maintain their habitual food intake and physical activity during the intervention period. These variables were evaluated with a 3-d food record for 2 weekdays and 1 weekend day over the 14-d intervention and by repeating the physical activity questionnaire in visit 3.
Table 2: The baseline characteristis didn't differ between the initially 2x33 subjects in the sucralose and control group, respectively (Romo-Romo 2018) - most importantly, the two-hour postload glucose load was virtually indentical (the fasting insulin, on the other hand, was n.s. higher in the sucralose group)
In addition, both groups were advised to avoid the consumption of any other products containing NNSs during the study. Adherence was evaluated with a specifc format in which participants registered the number of sucralose sachets consumed each day and the method of consumption" (Romo-Romo 2018, my emphasis).All subjects reported to the lab thrice: During the first visit the scientists tested the subjects for the most important exclusion criteria, i.e. having diabetes or prediabetes using the subjects' blood glucose concentration as their gauge (obviously, a glucose tolerance test would have been more accurate, but that's no critical methodological issue, because the baseline test would have revealed any subjects whose pre-diabetic status may have been overlooking).
Addendum: Do not fool yourself by assuming that the maltodextrin and dextrose in Splenda were to blame. Both were fully accounted for in the scientists' dietary analysis - with no inter-group differences in simple sugar intake (this has always been mentioned in the article, but a dozen of people must have overread it. That's why it's getting its own red (=important) infobox, now.
During visit 1, Romo-Romo et al. also assessed the subjects' physical activity, and food intake by the means of standardized questionnaires and took their anthropometric measures (weight, height, waist circumference, and hip circumference using measuring tapes), body composition (muscle and fat mass assessed via body impedance analysis (BIA)), and biochemical variables (fasting blood glucose, insulin, and lipid profile).The subjects'second visit at the laboratory was used to get a baseline reading on the FSIVGTT, i.e. the modified, frequently sampled intravenous-glucose-tolerance test and collect another round of 24-h dietary recalls. Together, the two food questionnaires from visit one and two served to establish the baseline energy and nutrient intake and assure that the subjects habitual non-nutritive sweetener consumption didn't exceed 5 servings per week.
"Bring in your empty and unused sachets, people!" - Is that adequate adherence control?
At their third visit to the lab, the study subjects returned the empty and unused sucralose sachets for the scientists to establish "adequate adherence". The latter was characterized by sufficient compliance (consumption >80% of the prescribed sucralose sachets) and persistence (consumption of sucralose sachets on >12 d of the 14-d intervention period). This may sound somewhat ridiculous, but it's common scientific practice. Moreover, even if we assume that this method isn't sufficiently accurate and (extreme case scenario) only 50% of the subjects actually consumed all 11 Splenda packs per day, this would only mean that even lower doses of sucralose can harm your insulin senitivity.
 |
| Figure 1: Changes in glucose metabolism variables in the intention-to-treat analysis (values didn't differ much for the per-protocol analysis); only the calculated insulin sensitivity showed sign inter-group differences (Romo-Romo 2018) |
What is important to note, here, is that, unlike the insulin sensitivity, the increased acute insulin response, as well as the disposition index and the glucose effectiveness, which worsened in both groups, didn't show the expected significant inter-group differences - a fact that could be a result of the high coefficient variance of the IVGTT, I discuss in the blue infobox below.
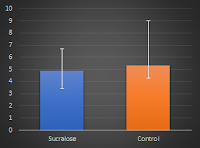 |
| Both groups started w/ a mean Si of 5.8 and ended up with 4.9 and 5.3x10-4/min/(mU/L), respectively - albeit with huge intra-group differences. |
Were the subjects indeed adherent or were there differences in their diets & activity?
Well, I would love to tell you, but ('crazy coincidence') the results of the food records, the subjects had to fill, are simply omitted from the full text of the study. The only thing we learn is that the adjustment of insulin sensitivity (Si) by "calories, and sugars consumed throughout the intervention" didn't affect the significance of the results (and yes, the extra dextrose and maltodextrin in Splenda was accounted for). So, if it's not dietary/activity differences the next logical question is:
Is the intravenous glucose test accurate and relevant in this context?
In view of the previously cited extreme intra-group differences, it is only logical to question the validity of the test of the FSIVGTT. According to the "American Diabetes Association" (1998) the FSIVGTT is yet one of two gold-standards (the other being the euglycemic insulin clamp) that 'satisfactorily' assess peripheral insulin resistance - 'satisfactorily' in this context means with a margin of error (CV) of 14-30% (Monzillo 2003). That may sound bad, but since we have to assume that these errors should be identical and random in both groups, they should cancel each other out for high enough participant numbers. Hence, the variability of the test results don't disqualify the results of the study at hand, they could, however, explain why the scientists weren't able to detect statistically significant effects of the other outcome measures.
So the test is great? Not so fast: What we shouldn't forget is a potential lack of real-world relevance compared to e.g. the oral glucose tolerance test (OGTT). After all, the FSIVGTT represents only the response to the appearance of glucose in the blood, not the more complex response that starts at the very moment your sweet taste receptors in the mouth detect the glucose solution you consume in an OGTT,... but I will save discussing that to the next info-box which will deal with the issue "Oral or intravenous - Which will provide more relevant results?".
- T2DM patients, for example, see improvements of the insulin sensitivity in the range of ~80% in response to treatment with a GLP-1 analogs (Zander 2002 | this study is also relevant because scientists speculate that the effects may be related to GLP1, the release of which is increased upon co-exposure of sucralose and glucose, Temizkan 2015), while ...
- sleep restriction acutely reduces the insulin sensitivity of healthy subjects by 13% per hour that's lost (the coefficient is based on reductions from 8 to 5 hours in Wong 2015), and
- two-weeks of HIIT training (15 min of exercise; 3x per week; 4–6 × 30-s cycle sprints) improves Si in healthy young men by 23% (Babraj 2009).
How likely is it that I am one of those in whom the insulin sensitivity declines, anyway?
If you take a look at the previously reported confidence intervals or the error bars in the figure in the "Is there any chance the reduced insulin sensitivity is irrelevant?"-infobox, you may be asking yourselves: "Would I be the guy with the 30% reduction in insulin sensitivity or the one whose insulin sensitivity wasn't affected, at all?" This is a question that relates to the relative risk of seeing significant reductions in insulin sensitivity in a single individual - the kind of statistical shenanigan you'll see way more often in epidemiological vs experimental studies. In the experimental study at hand, however, Romo-Romo et al. conducted a bunch of statistical analyses that may give us at least an estimate of your or rather the individual risk increase of the average normal-weight non-diabetic, normoglycemic man (21% body fat) or woman (28% body fat) in his or her early twenties who consumes high, but realistic amounts of sucralose over the course of two weeks (this is the point where I should remind you that this is the group of people to which the study results apply - and not to your 3-year-old niece, your diabetic granny or the Olympic gold medalist next door).
Don't underestimate your sucralose exposure. You are probably affected, too! If you think you're not consuming significant amounts of sucralose, take a look at the ingredient lists of your supplements. The label usually won't give you the numbers, a quick research in scientific papers, however, reveals that the amount of sucralose in 50g of commercially available flavored whey or casein proteins is probably in the range of 30-60mg (Pal 2010 & 2011, independent lab analyses); and you can expect similar concentrations in other sucralose sweetened supplements as well as "no-sugar" products you may be consuming on a per serving base (you can find an overview of products and the sweeteners they contain in Table 1).
In order to determine if dietary differences (which were non-significant when the scientists compared them directly) and other confounding factors such as changes in weight, physical activity, calories, and sugars consumed throughout the intervention may explain the deterioration of the subjects' insulin sensitivity, the scientists used a multiple linear regression model. However, even after the inclusion of "other variables that showed a tendency (P ≤ 0.20) in bivariate correlations with the percentage change for Si" (age, BMI, waist circumference, and percentage of body fat), the effect of sucralose was still significant. And..."[...t]he only variable that signifcantly modifed the Si was sucralose consumption (P = 0.02), with a β-coeffcient of −0.278 (95% CI: −0.419, −0.023) and R2 of 0.254 for this model; the other factors were not signifcant" (Romo-Romo 2018).Based on the model, the scientists also calculated the previously alluded to relative risk (RR) that a subjects' insulin sensitivity would decrease (comparing sucralose vs. no sucralose, no other parameters considered). The calculated RR for a worsening of the insulin sensitivity of a specific individual in the intervention group was 1.48 (95% CI: 1.00, 2.20; P = 0.04). In layman's terms, this means that the relative risk that his or her insulin sensitivity declined to any significant extent over the two week period was increased by 48%.
Don't forget: A 48% increase in relative risk can be a very small increase in absolute risk
Ha? That sounds huge, I know. Before you flush down all your sucralose sweetened pre-workout, post-workout, whey, and casein supplements down the toilette (don't ever do that, 'cause the sucralose and other sweeteners are also an environmental hazard :-), let's briefly check what this 48% increase may look like if it is expressed as your total relative risk of reductions in your precious insulin sensitivity - here's an example: Let's assume that your baseline risk that your insulin sensitivity will decline over the next to weeks is 2 in 1000, i.e. 0.2%. According to Romo-Romo's study, this 0.2% risk will increase by almost 50%, if you decide to consume 2.25g sucralose per kilogram body weight on a daily basis for two weeks. Now, as bad as that may sound, this does also imply that your absolute risk of a decline in insulin sensitivity increases by 'only' 0.3%. This is, by the way, not a totally unrealistic value, because we can safely assume that the insulin sensitivity of a healthy, lean, active individual like yourself who doesn't change either his/her dietary or activity patterns shouldn't change over the course of two weeks.
 |
| The increased insulin levels at t > 60 min in the OGTT suggests ill, the reduced insulin response in the IVGTT suggests beneficial effects of 4 weeks on 200mg of sucralose per day on glucose management (Letrit 2018). In theory, the OGTT has greater practical relevance, because few of us will inject glucose intravenously instead of eating CHO-rich foods, I guess. |
In view of what recent research tells us about the interaction of nutritively and non-nutritively sweetened foods with the taste receptors in the mouth, the GI tract, and on the pancreas as well as the modulatory role of the microbiome, the assumption that the IVGTT that was used in the study at hand, or similar intravenous tests (a euglycemic clamp, for example) would yield better, or let's say more relevant results than the good old oral glucose tolerance test (OGTT) becomes questionable.
And I am not the only one to question the practical relevance of intravenous glucose tolerance tests, in general, and the IVGTT, in particular. In an editorial comment which has been published at the same time as the study at hand, Pepino et al., who spearheaded the research into the potential negative effects of sucralose on human blood glucose management, point out that...
"[t]he IVGTT is [a] non-physiologic [test] because it omits important regulators of postprandial glycemia, including oral and intestinal sweet taste receptors in the gastrointestinal tract (which could likely be affected by regular consumption of sucralose)" (Pepino 2018).And guess what, a very recent study by Letrit et al. (2018), who used both an OGTT and IVGTT, confirms just that: You cannot assume to get identical results with an OGTT and IVGTT. And what's more, Letrit's study shows that the tests may even yield contradictory results (see Figure at the top left of this infobox). In their study, the scientists from Thailand wanted to test the effects of 4 weeks on 200 mg sucralose per day (vs. placebo capsules) in healthy volunteers who did not use non-nutritive sweeteners and found: (1) increased GLP1 and reduced insulin sensitivity in the OGTT (same results in obese subjects in Pepino 2013), but (2) a reduced acute insulin response at similar glucose levels when bypassing the taste receptors in mouth and GI tract in the IVGTT - this means that the OGTT suggested a reduced, the IVGTT, on the other hand, an increased insulin sensitivity.
GLP1 could be a mediating factor, here, but the Letrit study didn't assess it in the IV test
Needless to say that it will take more studies to assess, whether the differences Letrit et al. observed between their oral to intravenous test relate to the lack of taste receptor activation Pepino et al. mention in their editorial comment. If they do, however, the analysis of GLP1 levels in an IV-test in response to short-term sucralose administration would be the next necessary step to take in order to be able to explain what exactly was going on in the Letrit study (note: in the said study, the GLP1 levels were only measured in response to the OGTT, not during the FSIVGTT). Ultimately, this may also help us understand and estimate, how "dangerous" the regular consumption of sucralose actually is for healthy individuals.
Estimate: Only one in 116 subjects will definitely see significant reductions in insulin sensitivity
That sucralose does not pave the way to certain type II diabetes becomes even more obvious when we calculate another statistical value, the Number Needed to Treat (NNT), i.e. the number of people you would have to put on sucralose for one week to see a deterioration of the insulin sensitivity.
The question that remains is: What's the reason for the deterioration of the insulin sensitivity?
If we understood the mechanism, we would probably also be able to estimate the overall significance of the effect in terms of our glucose metabolism much better. Both, Letrit et al. and Pepino et al., seem to believe that the ill effects that were observed in the previously discussed studies (Letrit 2018 and Romo-Romo 2018) were - in one way or another - related to sucralose's modulatory effect on GLP1. Even if that were true, though, we still wouldn't know if this relationship is of a corollary or causal nature; and the contradictory results of Letrit's study rather complicate than aid in finding the underlying mechanisms by which sucralose exerts its medium-to-long-term effects on insulin sensitivity.
In view of the fact that I have another 66 studies related to sucralose, its safety and purported ill health effects to discuss (related to IBS, the microbiome, differential effects according to age and sex, the brain, blood pressure, body fat distribution, thyroid health, etc.), I will address other putative mechanisms in a general follow-up article about sucralose and leave you on the note that a recent (albeit very high dose rodent) study by Bornemann et al. (2018) points to what I currently believe could be an important finding when it comes to forming hypotheses about the health effects of sucralose: Unlike researchers have long believed, sucralose is metabolized in the gut; and what's even more disconcerting is that sucralose itself and its previously unknown (or ignored) metabolites can accumulate in mammalian tissue. These observations lead us back to my humorous "conspiracy theorist" comment from the introduction because a potential bioaccumulation of sucralose was not included in the "decision process for this agent and indicate that it now may be time to revisit the safety and regulatory status of this organochlorine artificial sweetener" (Bornemann 2018). In theory, it is thus (remotely) possible that Coke and Pepsi pulled the plug on sucralose just in time before (I apologize for using slanguage) 'the shit hits the fan'.
 |
| There will be a more general follow-up article on sucralose soon. If you don't want to miss it, head over to Facebook, Twitter, or Instagram and subscribe! |
Yes, there's the issue with the non-reported diet and physical activity data; and yes, there's the possibility that the IVGTT may miss important effects of sucralose's interaction with the sweet taste receptors in the mouth and intestinal tract (note: the ones on the pancreas would still be stimulated, so making the argument that there's no sweet taste receptor involvement, at all, in an IVGTT would not be valid).
Eventually, however, none of these 'shortcomings' negate the general validity of the scientist's conclusion that the use of high, but still realistic amounts of sucralose (2.25mg/kg body weight per day, i.e. 15% of the ADI) can negatively affect the insulin sensitivity of healthy individuals within only two weeks. And while this result is at least partially supported by the previously discussed (last infobox) results of Letrit's study, there's, much more research to be done to realistically assess the risk and relevance of these effects, the underlying mechanism, and related questions such as: Do these effects persist, if you stop using sucralose? Does the timing of the sucralose ingestion matter (w/ vs w/out meals)? Is there a linear, logarithmic, exponential, J- or J-shaped dose-response relationship? Or, will other parts of the general population (e.g. the elderly, toddlers and children, athletes, and diabetics) see similar, more pronounced or maybe no effects, at all?
While you're waiting for these studies to be conducted and published, it may, in fact, make sense to monitor and limit your personal sucralose intake. Unfortunately, the contemporary labeling regulations don't require that the food and supplement industry disclose the exact amounts of sucralose (and other sweeteners, aromas, etc.) in a product on the nutrition label. Hence, you will ultimately have to decide whether the (in absolute terms) small risk increase warrants cutting out all products that contain sucralose - a decision of which I hope that the article at hand will help you with. Ah, and I don't have to emphasize that replacing 100mg of sucralose in your diet with the 60g of sugar (sucrose) that produce the same sweet sensation wouldn't be the best strategy to avoid reductions in insulin sensitivity - do I? | Comment!
- American Diabetes Association. "Consensus development conference on insulin resistance: 5–6 November 1997." Diabetes Care 21.2 (1998): 310-314.
- Bornemann, Volker, et al. "Intestinal Metabolism and Bioaccumulation of Sucralose In Adipose Tissue In The Rat." Journal of Toxicology and Environmental Health, Part A (2018): 1-11.
- Babraj, John A., et al. "Extremely short duration high intensity interval training substantially improves insulin action in young healthy males." BMC endocrine disorders 9.1 (2009): 3.
- Buffini, Maria, et al. "Dietary intakes of six intense sweeteners by Irish adults." Food Additives & Contaminants: Part A 35.3 (2018): 425-438.
- Grotz, V. Lee, and Ian C. Munro. "An overview of the safety of sucralose." Regulatory toxicology and pharmacology 55.1 (2009): 1-5.
- Ha, Mi-Sun, et al. "Assessment of exposure of Korean consumers to acesulfame K and sucralose using a stepwise approach." International journal of food sciences and nutrition 64.6 (2013): 715-723.
- Lorenzo, Carlos, et al. "Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS)." Diabetes care 33.9 (2010): 2098-2103.
- Ma, Jing, et al. "Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects." American Journal of Physiology-Gastrointestinal and Liver Physiology 296.4 (2009): G735-G739.
- Monzillo, Lais U., and Osama Hamdy. "Evaluation of insulin sensitivity in clinical practice and in research settings." Nutrition reviews 61.12 (2003): 397-412.
- Spencer, Marisa, et al. "Artificial sweeteners: a systematic review and primer for gastroenterologists." Journal of neurogastroenterology and motility 22.2 (2016): 168.
- Pal, Sebely, Vanessa Ellis, and Suleen Ho. "Acute effects of whey protein isolate on cardiovascular risk factors in overweight, post-menopausal women." Atherosclerosis 212.1 (2010): 339-344.
- Pal, Sebely, and Vanessa Ellis. "Acute effects of whey protein isolate on blood pressure, vascular function and inflammatory markers in overweight postmenopausal women." British journal of nutrition 105.10 (2011): 1512-1519.
- Pepino, M. Yanina, et al. "Sucralose affects glycemic and hormonal responses to an oral glucose load." Diabetes care (2013): DC_122221.
- Pepino, M Yanina. "The not-so-sweet effects of sucralose on blood sugar control." The American Journal of Clinical Nutrition. 108.3 (2018):431-432
- Romo-Romo, Alonso et al. "Sucralose decreases insulin sensitivity in healthy subjects: a randomized controlled trial." The American Journal of Clinical Nutrition 108.3 (2018): 485-491.
- Schiffman, Susan S., and Kristina I. Rother. "Sucralose, a synthetic organochlorine sweetener: overview of biological issues." Journal of Toxicology and Environmental Health, Part B 16.7 (2013): 399-451.
- Temizkan, S., et al. "Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes." European journal of clinical nutrition 69.2 (2015): 162.
- Wong, Patricia M., et al. "Shorter sleep duration is associated with decreased insulin sensitivity in healthy white men." Sleep 38.2 (2015): 223-231.
- Zander, Mette, et al. "Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study." The Lancet 359.9309 (2002): 824-830.









