|
| You lose 600x more sodium than magnesium during a workout. The RDA is yet only ~3-4x higher (Montane. 2007). |
According to Sebastian et al. (2002) the latter is ~8-9:1 in other words: 8-9 mols of potassium per mol of sodium. That's miles apart from the 1:2-3 ratio the average Westerner (the exact ratio varies depending on which study you refer to) uses as a springboard to hypertension ;-)
The (un-)definite mineral synergism / antagonism chart
Another thing you may have noticed with yesterday's show is the fact that the show was pretty "topic centered". My personal feeling is that it has a much better flow this way and that not despite, but because Carl and I did not cover such a broad range of topics. I cherish the hopefully non-futile hope that you feel the same, but am obviously open for any constructive criticism from your side
Science Round-Up will air at 12PM EST, same URL as usual).
On that note, let's start with an "expansion" I already promised to deliver towards the end of the show: some information on the synergism and antagonism of the macrominerals. It's a pretty complex matter and the following illustration is based on generalizations. Some of them, like the low-level exception to the antagonism between calcium and magnesium, of which I believe that it is important to know are explicitly mentioned, others are not.
A very good example of the former, i.e. the important second order interactions is the influence sodium has on the antagonism between potassium and magnesium. The latter disappears, when sodium levels are high and magnesium is needed as a sodium antagonist. Similarly, the often-touted antagonism between magnesium and calcium is actually a co-factor relation, where any "antagonism" is only the result of imbalances between the two.
The good, the bad and the ugly: Just a question of the "wrong" perspective
One thing that should actually be obvious, but is often ignored in all the hoopla about the "good" and "bad" guys among the macro-minerals is that "antagonisms" do not contradict the essential nature of all of the electrolytes, which are - antagonistic or not - in the end all actors in the same metabolic play.
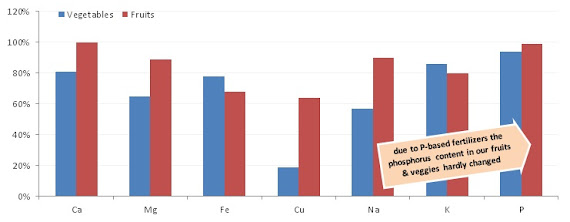
According to a 2009 paper by Dana Cordell et al. this may well change in the not all too distant future, after all "the quality of remaining phosphate rock is decreasing and production costs are increasing" (Cordell. 2009). With estimates saying that the demand for phosphorus is going to double within the next 40 years, it stands to reason that the decried overabundance of phosphorus, which is, among other things, also responsible for lowering the zinc content of the produce (cf. Peck. 1980) may be partly reversed within the next decades... I mean, we all know that nothing is as "convincing" as with financial interests, right?
The strong ion difference determines your pH levels
What's the difference between macro-minerals and their "little brothers" the trace minerals? Calcium, sodium, potassium, phosphorus, magnesium, chloride and sulfur are macro-minerals, because you need them in amounts that are greater than 100mg per day. Of the trace minerals, on the other hand you need less (in most cases much less) than 100mg per day. That does not mean though that Iron, zinc, copper, chroium, flouride, manganese, iodine, molybdenum and selenium were less important - it's merely a quantitative distinction.
While it stands to reason that there is a reason, calcium, sodium, magnesium and potassium are also called "electrolytes", astonishingly few people can actually give an ad hoc explanation why this is the case - and that despite the fact that their lives depend... no, not on the answer, but on the existence and physiological function of electrolytes ;-)If you have listened closely to your physics teacher, you will yet probably be aware that an "elecrolyte" (electro- ~ charge, -lyte ~ carrier) is a positively or negatively charged molecule (ion) and nothing out of the ordinary in nature.
In your body electrolytes are used to establish ionically charged gradients, similar to the gradient that exists between the positive and negative pole of a battery. These gradients are situated on the cell embranes in excitable tissues, such as muscle and verve, where they facilitate or hinder the influx / efflux of other charged particles.
One of these gradients, in fact probably the physiologically most significant one, by the way, is established by positive sodium (Na+) and potassium (K+) ions and their negative counterpart chloride (Cl-) - exactly those electrolytes you've heard about in yesterday's show (remember: whenever you hear "salt" it actually means Na + Cl).
The electrolytes are not the only charged particles ...
From your chemistry lessons you may remember that there are are not just ionic atoms, but also ionic molecules and that the electron configuration of these particles will determine how they bind, interact and react. But I guess, we have had more than enough complicated theory for today, so if you want to know how the anions and how the strong ion difference (SID) is calculated, check out this brief overview over at acid-base.com.
Rather than going into the details of the mechanism, I decided that it would probably of greater value to wrap the Seconds up with a brief overwiev of the downstream effects of a metabolic state, of which Pizzorno, Frassetto and Katzinger point out that it is not necessarily characterized by acidemia, i.e. pH levels below the "magic" (if we were honest, we'd you'd have to write arbitrary, here) cut-off limit of pH 7.35:
In other words, you don't have to suffer from diabetic or otherwise pathogenic "acidosis", to suffer from one of the following ill health-consequences:"Acidosis only becomes acidaemia when compensatory measures to correct it fail. To illustrate the difference between acidosis and acidaemia, take the following example: two processes occurring simultaneously in the same individual, such as a respiratory acidosis combined with a metabolic alkalosis. In this case, if the respiratory trend toward acidosis is greater than the metabolic trend, a pH of less than 7·35 may be reached, and would be considered acidaemia, despite the presence of a metabolic alkalosis. The intensity of each ‘process’ will determine the pH, but the terms themselves (acidosis, alkalosis) do not indicate a certain pH." (Pizzorno. 2009)
High intensity exercise can also lower your blood pH, an effect you can counter with sodium bicarbonate
Calcium loss, bone loss, osteoporosis - Unfortunately, this is not only the best known side effect of "being too acidic", it's also the only one people take serious. In that, scientists and laypress alike have zoned in on the high intake of animal proteins as the main confounding factor. But despite the fact that the high sulfur content (methionine, cysteine & co) does certainly contribute to the problem, the data in the figure at the right should make it quite clear that the stuff we eat and don't eat with our meats is at least as much to blame for the misery. In view of the fact that![]()
Hip fracture incidence per 100,000 study participants; aggregated data from cohorts from 33 countries (Frassetto. 2001) "[...] cereal grains themselves are net acid-producing and alone accounted for 38% of the acid load yielded by the combined net acid-producing food groups in the contemporary diet" (Sebastian. 2002)
the average (processed) grain addicted US citizen with his/her quasi non-existent vegetable intake would end up way on the left side of the x-axis of the graph on the right hand side, even if he ate not a single gram of animal protein - we would just have to relable the axis to "vegetable / acid forming food intake (including grains!)".- Increased renal nitrogen excretion and hampered protein synthesis - One of the less known effects of an increased acid/base ratio is an increase in nitrogen excretion that will obviously not simply hamper your gains, but can also set you up to sarcopenia (age-induced muscle loss).
In the end, the excretion of nitrogen is nothing, but an adaptive mechanism and a consequence of the catabolism of tissue protein. It is, if you will, a basic necessity for your body to rob your muscle and other tissue of glutamine and all other amino acids, that can be convert to glutamine in the liver, from where it is delivered to the kidney where it's used to synthesize ammonia and excrete the potentially toxic acid load. This will obviously mitigate the severity of the acidosis, it does yet also entail a net loss in muscle and organ protein that cannot be compensated for by an increase in acid forming protein in your diet.![]()
Correcting a diet-induced low grade metabolic acidosis with K-bicarbonate reduces the nitrogen loss of 750mg - 1000mg per day (per 60kg BW) in post- menopausal women (Frassetto. 1997)
As the data in the figure to the right goes to show you this is a process that's regulated on a day to day basis and the relief in nitrogen loss (data in mg/day/60kg) provided by bicarbonate supplementation (days 0-18) is transient and disappears as soon as you return to your regular low-base, high acid diet (days 19-30). - Impairments of the growth hormone / IGF-1 axes - Brunnger et al. tested in 1997 whether experimental acidosis would have an effect on the growth hormone / IGF-1 axis and observed a "significant decrease in serum IGF-1 concentration without a demonstrable effect on IGF binding protein 3", which points towards an acid induced "primary defect in the growth hormone/IGF-1 axis" that occurs "via an impaired IGF-1 response to circulating growth hormone with consequent diminution of normal negative feedback inhibition of IGF-1 on growth hormone" (Brunger. 1997). Interestingly, Mahlbacher et al. were able to show that the administration of IGF-1 can in turn ameliorate acidosis and thus correct the previously discussed nitrogen wasting (Mahlbacher. 1999).
In fact, potential physiological effects of the acid-induced impairment of the GH / IGF-1 axes had been observed much earlier, already. McSherry et al. for example report in a 1978 article in the Journal of Clinical Investigations that children with short stature and classic renal tubular acidosis developed normally, when they were treated with adequate amounts of alkalizing agents.Learn more about the effects of GH, IGF1 and it's splice variants MGF & co and their influence on skeletal muscle hypertrophy in the respective part of the Intermittent Thoughts on Building Muscle (go to the overview).
That similar negative effects can be observed even in the presence of "low-grade 'tonic' background metabolic acidosis" was confirmed by Frassetto et al. who observed statistically significant increases (+11%) in 24-hour mean growth hormone secretion in post-menopausal women with diet-induced low-grade metabolic acidosis, when their dietary acid load was neutralized with adequate amounts of potassium bicarbonate (Frassetto. 1997).
In a subsequently published study the scientists argue that the concomitantly observed increases in osteocalcin and bone metabolism would confirm the physiological significance of these changes (Frassetto. 2001). The effects on bone add to the well-known beneficial metabolic effects of growth hormone ( and line up with the recently reported association between low growth hormone levels and memory impairments (Wass. 2010).
In view of the bad press GH and IGF1 are getting, it is important to point out that we are talking about a normalization of the GH/IGF-1 axis, here. It is therefore unlikely that the restoration of a normal acid-base balance will have any of the anti-longevity and pro-cancerous (see next bulletin point) effects of growth hormone and IGF-1 you may have read about in the pertinent literature. - Potential protective / anti-cancer effects - While conclusive scientific evidence for the involvement of low-grade acidemia in the etiology of cancer is still missing, it has long been speculated that the genetic and epigenetic perturbations, which will turn normal cells into cancer cells may be triggered (among other factors) by disturbances in the acid-base equilibrium. As Ian Forrest Robey points out in his 2012 review of the literature, a diet induced
"[a]cid-base disequilibrium has has been shown to modulate molecular activity including adrenal glucocorticoid, insulin growth factor (IGF-1), and adipocyte cytokine signaling, dysregulated cellular metabolism, and osteoclast activation, which may serve as intermediary or downstream effectors of carcinogenesis or tumor promotion." (Robey. 2012)
If you want to learn more about the "state of the art research" on the potential link between latent dietary acidosis and the development of cancer, I suggest you simply read the free fulltext of the paper on PubMed.
If you adhere to these simple rules, there is no reason to be worried about "not getting your minerals" and other essential nutrients. After all, this is what distinguishes you from the "average" western omnivore, vegetarian or vegan who fails to meet most of his or her nutrient requirements (see figure to the right).
References:
- Brungger M, Hulter HN, Krapf R. Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: new cause of growth hormone in sensitivity in humans. Kidney Int. 1997; 51:216–221
- Cordell D, Drangert J-, White S. The story of phosphorus: Global food security and food for thought. Global Environ Change. 2009;19(2):292-305.
- DiMarino A. A Comparison Of Vegetarian Diets And The Standard Westernized Diet In Nutrient Adequacy And Weight Status. The Ohio State University. A Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Distinction from the School of Health and Rehabilitation Sciences of The Ohio State University. 2013.
- Frassetto L, Morris RC, Jr., Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in post-menopausal women. J Clin Endocrinol Metab. 1997: 82:254–259.
- Frassetto L, Morris RC Jr, Sellmeyer DE, Todd K, Sebastian A. Diet, evolution and aging--the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr. 2001 Oct;40(5):200-13.
- Mahlbacher K, Sicuro A, Gerber H, Hulter HN, Krapf R. Growth hormone corrects acidosis-induced renal nitrogen wasting and renal phosphate depletion and attenuates renal magnesium wasting in humans. Metabolism. 1999; 48:763–770
- May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986. 77:614–621.
- Mayer AM. Historical changes in the mineral content of fruits and vegetables. British Food Journal. 1997; 99(6):207 - 211
- McSherry E, Morris RC, Jr. At tainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest. 1978; 61:509–527.
- Montain SJ, Cheuvront SN, Lukaski HC. Sweat mineral-element responses during 7 h of exercise-heat stress. Int J Sport Nutr Exerc Metab. 2007 Dec;17(6):574-82.
- Peck NH, Grunes DL, Welch RM, MacDonald GE. Nutritional Quality of Vegetable Crops as Affected by Phosphorus and Zinc Fertilizers Agron. J. 1980; 72: 528–534.
- Pizzorno J, Frassetto LA, Katzinger J. Diet-induced acidosis: is it real and clinically relevant? Br J Nutr. 2010 Apr;103(8):1185-94.
- Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC Jr. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002 Dec;76(6):1308-16.
- Wass JA, Reddy R. Growth hormone and memory. J Endocrinol. 2010 Nov;207(2):125-6.
- Williams B, Layward E, Walls J. Skeletal muscle degradation and nitrogen wasting in rats with chronic metabolic acidosis. Clin Sci. 1991; 80:457–462