 |
| 2013's fitness craze was about Craze |
I guess, I would even have let the whole issue rest forever, if Mahmoud A. ElSohly and Waseem Gul hadn't published a peer-reviewed paper earlier this week that contains much more information than the previous short lab report ElSohly sent back to his clients over at Thermolife who had commissioned his lab to test a sample of DS Craze for amphetamines and decided that it was in everyone's best interest to publicize the results immediately.
Craze is not alone, Detonate& Assault join the party - but you already knew that, right?
The paper was published under advanced access in the Journal of Analytical Toxicology on December, 15 and the main result, i.e. the fact that the researchers "found an amphetamine-like compound that's not disclosed on their labels and puts athletes at risk of being banned from competition" (USAToday.com) did even make it into the online edition of USAToday.
What is phenethylamine? Phenethylamine (PEA) is a natural monoamine alkaloid that belongs to a class of chemicals with many compounds of known psychoactive and stimulant effects (Glen. 2005). It functions as a neuromodulator or neurotransmitter (Sabelli. 1976) with similar actions as amphetamine: Norepinephrine + dopamine release (Parker. 1988; Paterson. 1993). PEA and its substituted forms NDP and ETH is rapidly metabolized when taken orally (Shulgin. 1995-2009).
I guess by now few of you will be surprised that Craze was not the only supplement, of which ElSohly and his colleague, Waseem Gul, from the University of Mississippi found that it contains "undisclosed amounts" of "amphetamine like substances".What's the cause of the random fluctuations in "amphetamine-like" compounds
If you take a look at the data in Table 1, it does however become obvious that the total amount of "amphetamine-like" compounds in one cap of Gaspari Nutrition's fat burner / energy supplement Detonate is significantly lower than the varying amounts of N-ethyl-a-ethylphenethylamine aka ETH and phenethylamine aka PEA (also b-phenethylamine, or phenylethylamine) in a 5.5g serving of the tested LOTs of DS Craze - in the worst (or best?) case the latter contained ~80mg of either one or a combination of both agents.
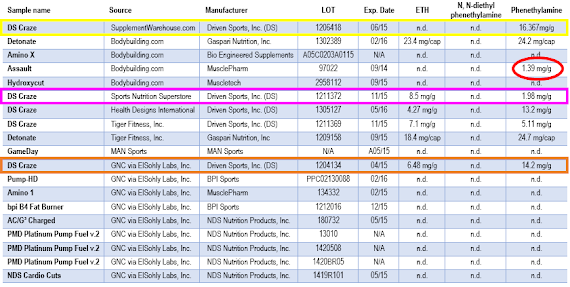 |
| Table 1: List of the analyzed products + ETH, phenethylamine & N, N-diethylphenethylamine contents (ElSohly. 2013) |
The source of "contamination"is actually a source of contamination, i.e. PEA and ETH occur randomly in some batch of raw ingredients DS got from God knows where in China.![]()
On a side note: A big belly is a greater threat to our brains than Craze | more - The assay the researchers developed is not accurate, or at least not as accurate as their (albeit sophisticated) tests would suggest, i.e. the day-to-day variability was significantly higher than the 9.8%, and 3.1% the researchers measured using a low and high dose control sample.
- Someone at DS was super smart and thought: "If the effects of these ingredients are similar, I will just randomize the exact dosage, to make sure that it looks as if it was accidentally contaminated".
What? Oh, yes, I start gossiping, again. Sorry! But that's just human; or, as R.I.M. Dunbar from the University of Liverpool has it, it's evolutionary preserved and an "important a component of human interaction" and displays "a mechanism for bonding social groups, tracing these origins
back to social grooming among primates." (Dunbar. 2004)
 |
| PEA Alternatives: "Theanine or Caffeine? Soda, Black or Green Tea? What's Going to Get Your Brain Going?" | more |
As far as I know nobody got physically harmed and the amounts of PEA and ETH in Craze are not exactly so high that you'd have to be afraid that you could have killed a couple of brain cells with it. And unless he or she found a way to inject the PEA equivalent of ~100g of Craze right into his /her veins, it's unlikely that he or she'd die (the figure is calculated based on the LD50 in rodents, so I would not rely on its accuracy; cf. Lands. 1952 ;-) ...
Don't get me wrong, I don't intend to play the whole case down, but let's not get carried away, here.- Dunbar, R. I. (2004). Gossip in evolutionary perspective. Review of general psychology, 8(2), 100.
- ElSohly, M. A., & Gul, W. (2013). LC–MS-MS Analysis of Dietary Supplements for N-ethyl-α-ethyl-phenethylamine (ETH), N, N-diethylphenethylamine and Phenethylamine. Journal of Analytical Toxicology, bkt097.
- Glen, R., Hanson, P.J., Venturelli, A., Fleckenstein, E. (2005) Drugs and Society.9th edition. Jones and Bartlett Publishers: Sudbury, MA. ISBN 978-0-7637-3732-0 Retrieved 2011-04-19.
- Lands, A. M., & Grant, J. I. (1952). The vasopressor action and toxicity of cyclohexylethylamine derivatives. Journal of Pharmacology and Experimental Therapeutics, 106(3), 341-345.
- Parker, E. M., & Cubeddu, L. X. (1988). Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding. Journal of Pharmacology and Experimental Therapeutics, 245(1), 199-210.
- Paterson, I. A. (1993). The potentiation of cortical neuron responses to noradrenaline by 2-phenylethylamine is independent of endogenous noradrenaline. Neurochemical research, 18(12), 1329-1336.
- Sabelli, H. C., Mosnaim, A. D., Vazquez, A. J., Giardina, W. J., Borison, R. L., & Pedemonte, W. A. (1976). Biochemical plasticity of synaptic transmission: a critical review of Dale's Principle. Biological psychiatry, 11(4), 481-524.
- Shulgin, A., Shulgin, A.Erowid Online Books: ‘PIHKAL’ - #142 PEA. Transform Press: Berkeley, CA. < http://www.erowid.org/library/books_online/pihkal/pihkal142.shtml > (20 Dec 2013, date last accessed)

