![]() |
| Will a small cup of coffee ruin the benefits you can derive from creatine supplementation? Sounds impossible, but it's a die-hard rumor with surprising scientific backing. Now, a new study could finally settle the debate. |
As topic for the thesis he submitted to the faculty of the
University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Master of Arts at the
Department of Exercise and Sport Science (Exercise Physiology), Eric T. Trexler selected the "Effects of Creatine, Coffee, and Caffeine Anhydrous on Strength and Sprint Performance" (Trexler. 2015).
This is, as some of you will immediately recognize, at least in parts, a variation of the age-old question, whether the purported diuretic effects of caffeine would impair the proven ergogenic effects of creatine. In that, it's a variation, because the domain of concern is not - as it is for most bros - solely restricted to resistance training, but extends beyond the investigated one-rep max on the leg press and into the realms of sprint performance. But let's tackle things one after the other.
You can learn more about creatine at the SuppVersity ![]()
Creatine Doubles 'Ur GainZ!
![]()
Creatine, DHT & Broscience
![]()
Creatine Better After Workout
![]()
ALA + Creatine = Max Uptake?
![]()
How is Creatine Metabolized?
![]()
Build 'Ur Own Buffered Creatine
As Trexel points out, the study at hand "sought to directly compare effects of caffeine-matched (300 mg) doses of caffeine anhydrous [CAF | that's basically the same stuff you will have in your pre-workout] and coffee [COF | that's ~3 cups of the beverage that many of you will be drinking on a daily basis] on strength
and sprint performance, and to determine if CAF or COF intake modulate the effects of creatine (CRE) loading" (Trexel. 2015 | my
emphasis).
Now, you propably don't need a PhD to be able to tell that
creatine and
caffeine are currently among the most popular and best proven nutritional ergogenic aids. What is odd, though, is that supplement companies have made a habit of packing both into one product, even though there's the long-standing suspicion that caffeine may blunt the effects of creatine.
![]() |
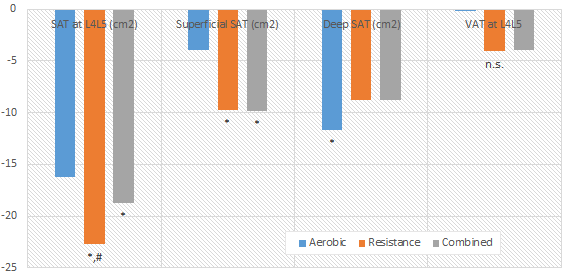
| Figure 1: Caffeine blunts the beneficial effects of creatine loading on dynamic torque production (Vandenberghe. 1996). |
And there are in fact a handful of people who still believe this was a huge mistake - mostly, because early studies into the concomitant administration of both agents like a Vandenberghe et al. (1996) clearly indicate that "caffeine counteracts the ergogenic action of muscle creatine loading" by (in this particular study) blunting the increase in dynamic torque production. This study, as well as a study by Hespel et al. who observed opposing effects of creatine and caffeine on the relaxation time of skeletal muscle (creatine decreases it, while caffeine increases it), are reason enough for skeptics like Tarnpolsky et al. (2010) to say that "there is no rationale for their simultaneous use" even though they have to admit that evidence is inconclusive:
"Although there is little rationale for taking both caffeine and creatine simultaneously as ergogenic aids, some have reported that the acute consumption of both negated the ergogenic benefits." (Tarnpolksy. 2010).
On the other hand, there are good reasons to be skeptical about the implications of the Vandenberghe and Hespel studies, too. It must be taken into consideration, for example that...
![]() |
| When is the best time to take your creatine supplement? Before or after your workouts? Learn more in this Suppversity Classic: "Lean Mass Advantage of Post- vs. Pre-Workout Creatine Supplementation Confirmed. Older Trainees Benefit Most" | more. |
the short term creatine loading protocol used in Vandenberghe's study is no longer recommended and the assumption that caffeine + creatine won't mix in the long-run would require studies that investigate that in the long run,- the study cross-over design of Vandenberghe's study in which the subjects received all three treatments in random order incorporated a 3-week washout period that was as follow up studies on the long-lasting effects of creatine supplementation suggest probably insufficient for the creatine levels to return to normal; after all, the minimal washout period for creatine is estimated to be ~4 weeks (Hultman. 1996)
- the Vandenberghe study used only one exercise to test the effects and does therefore hardly reflect the effects on real-world athletic performance,
- lastly, many researchers have dismissed a potential interaction between creatine and chronic caffeine ingestion, because some of the early creatine studies with highly beneficial results have administered it dissolved in coffee/tea (Greenhaff. 1993; Birch. 1994); so have more recent studies that in which the effects of one of the previously mentioned nutraceuticals were tested (Smith. 2010; Lowery. 2013)
Against that background and in view of the fact that none of the long(er) term creatine supplementation studies that dominate the scientific landscape of the late 20th and early 21st century reports that coffee connaisseurs wouldn't let alone couldn't benefit from creatine supplements (Fukuda. 2010; Smith. 2010), the results of Vandenberghe's study would have to be reproduced even if there was no contradictory evidence from a 1998 study by Vanakosk et al. who observed no interaction between creatine and caffeine in their crossover study (Vanakosk 1998).
Where's the acute phase study data? While Trexel did an acute phase and a chronic supplementation study, I will not discuss the results of the acute phase study in detail, because the only effects Trexel observed in this part of the his experiment were (unsurprisingly) the well-known beneficial effects of caffeine on acute exercise performance and even those were ... well, let's just say 'surprisingly inconclusive' - which means that I have seen much more significant benefits from caffeine in different experimental contexts (see Astorino. 2010 for a review).
To eventually find out if thousands of athletes competing in both aerobic and anaerobic sports are making a mistake, when they're pounding commercially prepared or self-made creatine + caffeine concussions, the initially mentioned study by Trexel was designed to find out if
chronic coffee or caffeine anhydrous consumption blunts the ergogenic effect of creatine loading on strength and sprint performance outcomes.
Using subjects with previous resistance training experience, Trexel determined if caffeine (CAF) or coffeee (COF - both at
300mg caffeine per serving) intake modulates the effects of creatine (CRE) loading with
20 g/day, split between 4 servings by conducting the same battery of strength and sprint performance tests before and after the acute and chronic supplementation with coffee, creatine or caffeine or a combination of both (CRE + CAF or CRE + COF).
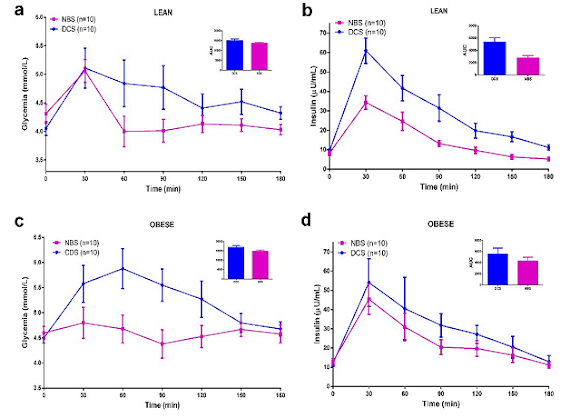
Unfortunately, the results of the by all means well-designed study are not clear enough to settle the debate once and for all even though, no inhibitory effects of caffeine or coffee on the ergogenic effects of creatine were observed. Personally, I'd say, though, that the total evidence would suggest that if there is an inteference, it's probably negligible in the long run.But let's get back to the study at hand and what it tells or rather doesn't tell us about a possible interference: As you can see in
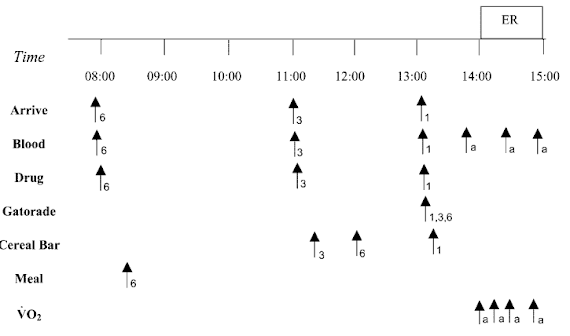
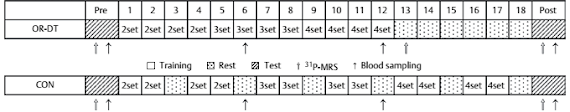
Figure 2, Trexel's study does not suffer from the same problems as the previously cited study by Vandenberghe. It's not a cross-over study, so too little washout time is not a problem. On the other hand, the number of subjects (13-14 in each group) is not exactly high enough to make sure that the differences you're expecting will be significant if you happen to have selected a few creatine non- or hyper-responders that mess with your data.
![]() |
| Figure 2: Overview of the study design for the chronic supplementation study (Trexel. 2015) |
An additional problem is the total length of the study. With
only 5 days, we are - once again - not in the practically more relevant longer-term chronic supplementation time-frame that would mimic the way most of you are probably using their creatine products. Yes, previous studies have shown significant effects even in a time-frame as short as that, but you will also know that creatine is not only one of the few supplements where the effects may accumulate over weeks, but also one of the few where long-lasting effects of previous (even way beyond the previously cited wash-out period) can be safely excluded. Against that background one could (I know you're always smarter after you've done a study, so no smart-assing here) argue that it
may not have been enough to pick subjects that had not taken creatine in the last three moths, but were not necessarily creatine naive.
What distinguishes creatine responders from non-responders? This question has still not been satisfactorily answered, but evidence from a 2004 study by Syotuik et al. indicates that different baseline creatine levels, the total muscle mass and the ratio of fast- to slow-twitch may determine whether you're going to see huge gains or no effect at all. To be more precise, Syotuik et al.'s observation suggest that ideally, you'd have a low baseline creatine level, lots of lean muscle and a high number of fast-twitch muscle fibers.
Yes, I know, being "creatine naive" does not sound like it could be important, but but in view of the anecdotal evidence that no "creatine cycle is as effective as the first one" as well as the scientific evidence that one's baseline creatine levels have a significant effect on whether you "respond" or "don't respond" to supplementation (Syrotuik. 2004 | see red box, as well), it
could at least partly explain why the results of the study are somewhat inconclusive.
![]() |
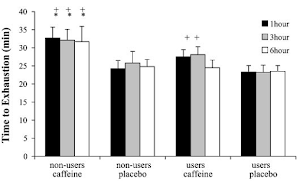
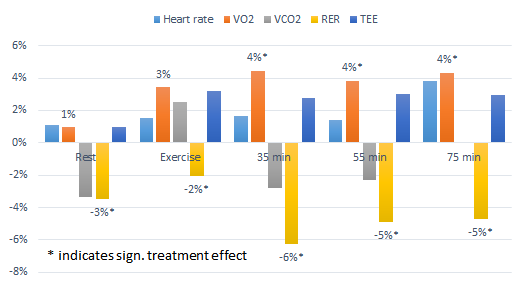
| Figure 3: If we go by serum creatine levels it would seem as if caffeine wasn't a problem - specifically if it comes from coffee. Unfortunately, serum creatine is pretty irrelevant and the performance data in form of changes in total work and the number of reptitions on the leg press and bench showed no signifant intergroup difference. Now this would suggest that it's not problem to take creatine with coffee or caffeine, but convincing evidence that caffeine does not impair the beneficial effects of creatine would require these beneficial effects to show (data after chronic suppl. from Teaxel. 2015). |
And with "inconclusive" I am attributing the data in
Figure 3 which tells you - more than anything else - that
neither creatine
norcaffeine produced a significant performance increase in any of the relevant parameters over the five-day study period.
Only the increase in plasma creatine was statistically significant. That's nice, because it shows that mixing your creatine into instant coffee, which is what the guys in the coffee + creatine group were told to do, appears to rather boost than hamper the intestinal absorption of creatine. Eventually, however, this information is irrelevant, because it is the increase in intramuscular phosphocreatine stores that's driving the (not observed) performance benefits - not an increases in serum creatine.
![]() |
| Another die-hard creatine myth based - just like the myth from the study at hand - on the results of a single study (and this time without rational hyopthesis to explain the results) is that creatine would increase DHT and thus trigger hair loss and prostate cancer | Learn more about this bogus. |
So are we left with nothing? Not exactly, after all, the study confirmed that caffeine and coffee both have their merit as acute phase ergogenics. It's correct, though, that this does not tell us, if caffeine will blunt the beneficial effects of creatine in either the short or the long run. In this respect, we are thus about as wise as before; and that in spite of the fact that Trexel is obviously right when he writes that "[t]he addition of CAF and COF did not appear to influence performance outcomes of CRE supplementation" (Trexel. 2015). In view of the fact that no significant effects of creatine supplementation were observed, we still need future long(er) term studies that combine coffee / caffeine + creatine and tests it against placebo and creatine alone - maybe even in different dosages - to answer the question whether it does or doesn't matter if you consume caffeine when you're "on" creatine once and for all |
Comment on FB!
References:
- Astorino, Todd A., and Daniel W. Roberson. "Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: a systematic review." The Journal of Strength & Conditioning Research 24.1 (2010): 257-265.
- Birch, R., D. Noble, and P. L. Greenhaff. "The influence of dietary creatine supplementation on performance during repeated bouts of maximal isokinetic cycling in man." European journal of applied physiology and occupational physiology 69.3 (1994): 268-270.
- Fukuda, David H., et al. "The possible combinatory effects of acute consumption of caffeine, creatine, and amino acids on the improvement of anaerobic running performance in humans." Nutrition research 30.9 (2010): 607-614.
- Greenhaff, Paul L., et al. "Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man." Clinical Science 84 (1993): 565-565.
- Hespel, Peter, B. Op‘t Eijnde, and Marc Van Leemputte. "Opposite actions of caffeine and creatine on muscle relaxation time in humans." Journal of Applied Physiology 92.2 (2002): 513-518.
- Hultman, E., et al. "Muscle creatine loading in men." Journal of applied physiology 81.1 (1996): 232-237.
- Lowery, Ryan P., et al. "Effects of 8 weeks of Xpand® 2X pre workout supplementation on skeletal muscle hypertrophy, lean body mass, and strength in resistance trained males." J Int Soc Sports Nutr 10.1 (2013): 44.
- Smith, Abbie E., et al. "The effects of a pre-workout supplement containing caffeine, creatine, and amino acids during three weeks of high-intensity exercise on aerobic and anaerobic performance." J Int Soc Sports Nutr 7.10 (2010): 10-1186.
- Syrotuik, Daniel G., And Gordon J. Bell. "Acute Creatine Monohydrate Supplementation: Adescriptive Physiological Profile Of Responders Vs. Nonresponders." The Journal Of Strength & Conditioning Research 18.3 (2004): 610-617.
- Tarnopolsky, Mark A. "Caffeine and creatine use in sport." Annals of Nutrition and Metabolism 57.Suppl. 2 (2010): 1-8.
- Trexler, Eric T. Effects of creatine, coffee, and caffeine anhydrous on strength and sprint performance. Diss. THE UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL, 2015.
- Vanakoski, Jyrki, et al. "Creatine and caffeine in anaerobic and aerobic exercise: effects on physical performance and pharmacokinetic considerations." International journal of clinical pharmacology and therapeutics 36.5 (1998): 258-262.
- Vandenberghe, K., et al. "Caffeine counteracts the ergogenic action of muscle creatine loading." Journal of applied physiology 80.2 (1996): 452-457.


![]()